Had to Atop Humira Will It Work Again
A black box warning in Humira'due south characterization highlights the chance of serious infections leading to hospitalization or death, including TB, bacterial sepsis, invasive fungal infections and infections due to opportunistic pathogens. It besides features cancers, notably lymphoma and hepatosplenic T-jail cell lymphoma.
Other warnings listed in Humira'south label include severe allergic reactions, hepatitis b reactivation, neurological reactions, blood reactions, worsening congestive center failure and lupus-like syndrome.
Common Side Furnishings
I of the most common side furnishings of Humira is a reaction at the injection site. The U.South. Food and Drug Administration received reports of more than 10,000 cases of injection-site reactions related to the medication in 2014. Injection-site reactions include redness, rash, swelling, itching or bruising. Symptoms typically go away within a few days. Even so, patients should phone call a doctor immediately if they have hurting, redness or swelling around the injection site that doesn't get away within a few days or gets worse.
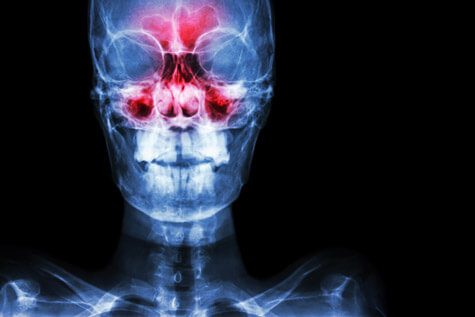
Sinusitis and upper respiratory infections are among the about unremarkably reported side effects of Humira.
During clinical trials involving nearly ane,400 people, 17 percent of patients treated with Humira reported upper respiratory infection compared with 13 percent of people who received a placebo. Another eleven percent of Humira-treated patients reported sinusitis while just nine percentage of people given the placebo reported the status.
Headache and rash were each reported past 12 percent of Humira-treated patients. Eight percent and 6 per centum of placebo-treated patients reported headache and rash, respectively.
| Side Effects | Humira (705 patients) | Placebo (690 patients) |
|---|---|---|
| Upper Respiratory Infection | 17% | 13% |
| Sinusitis | 11% | 9% |
| Headache | 12% | 8% |
| Rash | 12% | 6% |
Other reported side furnishings were flu syndrome, nausea, abdominal hurting, abnormal laboratory tests, high amounts of cholesterol in the blood, high levels of fat particles in the blood, blood in urine, increased alkaline phosphatase, accidental injury, back pain, urinary tract infection and hypertension.
Serious Infections
Serious and fifty-fifty fatal infections are a recognized risk of Humira. These serious infections may be caused by viruses, fungi or leaner. They tin can spread throughout the body and may atomic number 82 to hospitalization and death.
Fact
The take a chance of serious infection may be even greater in patients who utilize Humira at the same fourth dimension as other biologics.
The take chances of serious infection may be even greater in patients who utilize Humira at the same fourth dimension as other biologics, including Orencia (abatacept), Kineret (anakinra), Remcade (infliximab), Enbrel (etanercept), Cimzia (certolizumab pegol) or Simponi (golimuma). Some patients who were treated with rituximab (Rituxan) followed by TNF blockers also showed a higher rate of serious infections.
In addition to a tuberculosis (TB) alarm, today'due south Humira label carries a boxed alarm for invasive fungal infections, such as histoplasmosis, as well as Legionella and Listeria infections. Simply that was not always the case. For years, Humira's black boxed warning merely included the hazard of TB.
Tuberculosis (TB)
Humira'due south black box warning clearly advises patients be tested for TB earlier starting Humira. Patients who exam positive for TB should start treatment for the disease prior to starting Humira. Doctors should monitor all patients closely for signs and symptoms of TB during treatment with Humira, even if the initial latent TB test is negative.
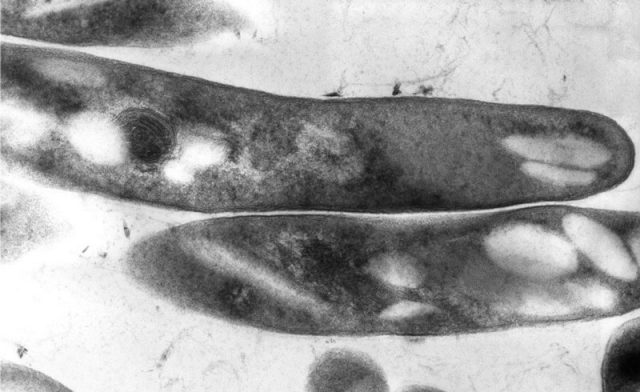
Transmission electron microscopic (TEM) paradigm of M.tuberculosis bacteria
"Cases of reactivation of tuberculosis and new onset tuberculosis infections have been reported in patients receiving Humira, including patients who have previously received treatment for latent or active tuberculosis," co-ordinate to the FDA.
Symptoms related to TB include a coughing, fever, weight loss or loss of torso fat and muscle. People considering Humira should let the prescribing doctor know if they have TB or have been in close contact with someone with TB. Also let the doc know if the patient was built-in in, lives in or traveled to a place where in that location is more risk for getting TB.
Fact
In 2015, a Central Florida human being filed a lawsuit, claiming he became paralyzed after using Humira.
According to the lawsuit, the human was given a shot of Humira and before long thereafter began feeling numb from the waist downwardly. He was then diagnosed with TB, according to the accommodate.
Invasive Fungal Infections
In September 2008, the FDA appear it would require makers of TNF blockers, like Humira, to highlight information about the take chances of invasive fungal infections in a blackness box alert likewise equally in the warnings section of the drug's prescribing information.
The agency notified health care professionals that it had received reports of people developing pulmonary and disseminated histoplasmosis, coccidioidomycosis, blastomycosis and other infections while taking TNF blockers, such as Humira, and doctors were not consistently identifying the infections.
"In some patients, the diagnosis of histoplasmosis was initially unrecognized and antifungal treatment was delayed. Some of these patients died from histoplasmosis. At that place were as well deaths in patients with coccidioidomycosis and blastomycosis."
Legionella and Listeria Leaner
In September 2011, the FDA appear it had added information about infection with Legionella and Listeria bacteria to the black box alarm for all TNF blockers, including Humira.
The bureau explained it had identified cases of Legionella pneumonia in people treated with TNF blockers. The FDA searched its Agin Effect Reporting Arrangement and identified reports of 80 patients who developed Legionella pneumonia between 1999 and 2010 after taking Humira or other TNF blockers. At least fourteen people died.
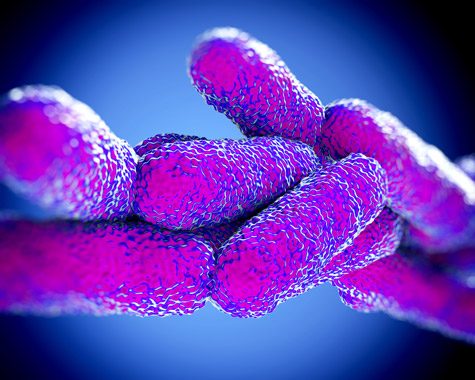
Close-up illustration of Legionella Pneumophila bacteria
In addition, the FDA searched English-language medical literature and identified instance reports of 23 patients who developed Legionella pneumonia afterwards existence treated with TNF blockers. Four patients experienced severe pneumonia requiring mechanical ventilation; five patients received treatment in a hospital intensive-care unit of measurement and 3 patients died.
The FDA has also received reports of people taking TNF blockers and developing serious infections due to Listeria monocytogenes. The agency searched English-language medical literature and identified 26 published cases of Listeria infection in patients treated with TNF blockers. Infections included meningitis, bacteremia, endophthalmitis and sepsis. At that place were seven reported fatalities.
"In addition, FDA identified fatal Listeria infections in a review of data regarding laboratory-confirmed infections that occurred in pre-marketing phase ii and phase iii clinical trials and from post-marketing surveillance," the agency said.
Legionella and Listeria Take chances
Many of the patients who developed Legionella pneumonia or Listeria infections had received immunosuppressive drugs, such as methotrexate and corticosteroids, at the same fourth dimension as a TNF blocker.
Cancer
Children and adults taking Humira may accept an increased chance of getting cancer. Specifically, patients taking TNF blockers have adult lymphoma, pare cancer (including basal prison cell and squamous cell) and leukemia. Some children, teenagers and immature adults have adult hepatosplenic T-cell lymphoma, a rare, often fatal cancer of white blood cells.
Lymphomas, Leukemia and Melanoma
In June 2008, the FDA announced it was investigating 30 reports of cancer in children and young adults who were treated with TNF blockers for Juvenile Idiopathic Arthritis, Crohn'due south disease or other diseases.
Fact
Use of TNF blockers is associated with an increased take a chance of lymphoma and other cancers in children and adolescents.
Reports of cancer were submitted to the FDA's Adverse Issue Reporting Arrangement over 10 years, beginning in 1998 — after the offset TNF blocker hit the market — through April 29, 2008. The cases involved children and young adults who took TNF blockers with methotrexate, azathioprine or 6-mercaptopurine.
Nigh half the cancers were lymphomas, including both Hodgkin's and non-Hodgkin'southward lymphoma; other cancers reported included leukemia, melanoma and solid organ cancers.
In a follow-up declaration in Baronial 2009, the FDA said it had completed its analysis and concluded that at that place is an increased take a chance of lymphoma and other cancers associated with the use of TNF blockers in children and adolescents. The bureau added the new rubber information to the boxed alert for Humira and the other TNF blockers.
In add-on, the FDA added new warnings about leukemia and new-onset psoriasis in patients treated with TNF blockers.
Hepatosplenic T-cell Lymphoma
The FDA put out 2 alerts in 2011 regarding TNF blockers and the development of cancer. First, in April, the FDA said information technology continued to receive reports of hepatosplenic T-cell lymphoma (HSTCL).
The reports were primarily in adolescents and immature adults existence treated for Crohn'due south disease and ulcerative colitis with TNF blockers also as azathioprine and/or mercaptopurine. One patient was existence treated for psoriasis and 2 patients were beingness treated for rheumatoid arthritis.
The FDA reviewed data from the Adverse Effect Reporting System database, literature and the HSTCL Cancer Survivors' Network and identified two cases of HSTCL with Humira and five cases with the combination of Humira and Remicade.
The cases were reported between the time TNF blocker marketing started and December 31, 2010. Of the v cases identified with the combination of Remicade and Humira utilise, iv involved concomitant employ of mercaptopurine or azathioprine.
FDA Announces 'Enhanced Safe Surveillance' for Humira
In a second 2011 safe communication, the FDA appear that information technology is requiring manufacturers of TNF blockers to "perform enhanced rubber surveillance" for TNF blockers.

Enhanced surveillance allows the FDA to more than completely capture and clarify malignancies in pediatric patients
As part of the enhanced safe surveillance, manufacturers must behave in-depth follow-upwardly of reports of cancer cases and submit all reports of cancer in pediatric and immature adult patients to the FDA inside 15 days of becoming aware of the report. The manufacturers must likewise provide annual summaries and assessments.
"This type of safety surveillance is important for our improved understanding of malignancies in pediatric and young adult patients treated with TNF blockers considering it volition let [the] FDA to more than completely capture and clarify all reported malignancies based on more complete and consistent reports," the FDA said.
The agency says it will periodically re-evaluate the enhanced surveillance requirement through 2021.
Astringent Allergic Reactions
People have experienced anaphylaxis (astringent, life-threatening allergic reaction) and angioneurotic edema (swelling) post-obit Humira administration.
Fact
Vi percent of children with JIA and v per centum of children with Crohn'due south reported allergic reactions with Humira use during studies.
In clinical trials of Humira in adults, patients suffered allergic reactions, including allergic rash, anaphylaxis and urticarial (skin rash).
In a written report of Humira in children with Juvenile Idiopathic Arthritis, six per centum of patients suffered allergic reactions — including allergic rash — within the first 48 weeks of treatment. About v pct of patients in a study of Humira in children with Crohn'southward disease suffered allergic reactions.
Signs of a serious allergic reaction include hives, trouble breathing, and swelling of the confront, eyes, lips or mouth. Patients should call their physician or get medical help immediately if they feel symptoms of an allergic reaction.
Hepatitis B Reactivation
People who carry hepatitis B virus (HBV) in their blood should be extra cautious when deciding whether to employ Humira considering the virus can go active during treatment.
"In some instances, HBV reactivation occurring in conjunction with TNF blocker therapy has been fatal," according to Humira's label. "The majority of these reports have occurred in patients concomitantly receiving other medications that suppress the immune organization, which may too contribute to HBV reactivation."
Patients should undergo claret tests earlier, during and for several months after treatment with Humira.
It's important to let the prescribing doctor know if the patient suffers from any of the following symptoms of a possible hepatitis B infection:
- Muscle aches
- Clay-colored bowel movements
- Feeling very tired
- Fever
- Dark urine
- Chills
- Skin or optics look yellowish
- Stomach discomfort
- Piffling or no appetite
- Pare rash
- Vomiting
Neurological Reactions
Humira's label warns consumers that the medicine has been associated with fundamental and peripheral nervous system demyelinating disease. This includes multiple sclerosis (MS), optic neuritis and Guillain-Barré syndrome.
Some people experienced symptoms of these disorders for the starting time fourth dimension following Humira use; others suffered from worsening of symptoms they already had.
"Exercise caution in because the use of HUMIRA in patients with preexisting or recent-onset central or peripheral nervous system demyelinating disorders," the Humira label states.
Claret Reactions
Though rare, some people have experienced blood reactions with use of TNF blockers, including Humira.
Humira's label specifically warns of reports of pancytopenia, a condition in which the body has besides few red blood cells, white blood cells and platelets.
Blood reactions reported with TNF use include:
- Aplastic anemia
- not enough new blood cells
- Thrombocytopenia
- not plenty platelets in the blood
- Leukopenia
- not enough white blood cells
Signs and symptoms that may indicate blood dyscrasias or infection include persistent fever, bruising, bleeding and pallor. Anyone who experiences these symptoms while using Humira should contact a doctor.
Heart Failure
Humira may cause new center failure or worsening of heart failure patients already accept.
The drug's label warns of cases of worsening congestive eye failure (CHF) with Humira, though the medicine has not been formally studied in patients with the condition.
Fact
Though the drug has not been formally studied in patients with the condition, Humira'due south characterization warns of cases of worsening congestive middle failure.
Other Humira Warnings
Humira may crusade immune reactions, including a lupus-similar syndrome. Symptoms may improve when patients stop taking Humira, though they should not stop treatment without consulting a physician. Symptoms include chest discomfort or pain that does not go away, shortness of breath, joint hurting or a rash on the cheeks or arms that gets worse in the sun.
In add-on, liver bug — including ones that tin can lead to liver failure and expiry — can happen in people who use TNF blockers, such equally Humira. Symptoms of liver bug include fatigue, yellow-looking skin or eyes, poor appetite or airsickness, and pain on the right side of your stomach.
Some Humira users suffered new psoriasis or worsening of psoriasis they already had. Signs of psoriasis include ruddy scaly patches or raised pus-filled bumps.
Source: https://www.drugwatch.com/humira/warnings/
0 Response to "Had to Atop Humira Will It Work Again"
Post a Comment